Bridging Pharmaceuticals and Experts
A Platform Uniting Innovation and Expertise
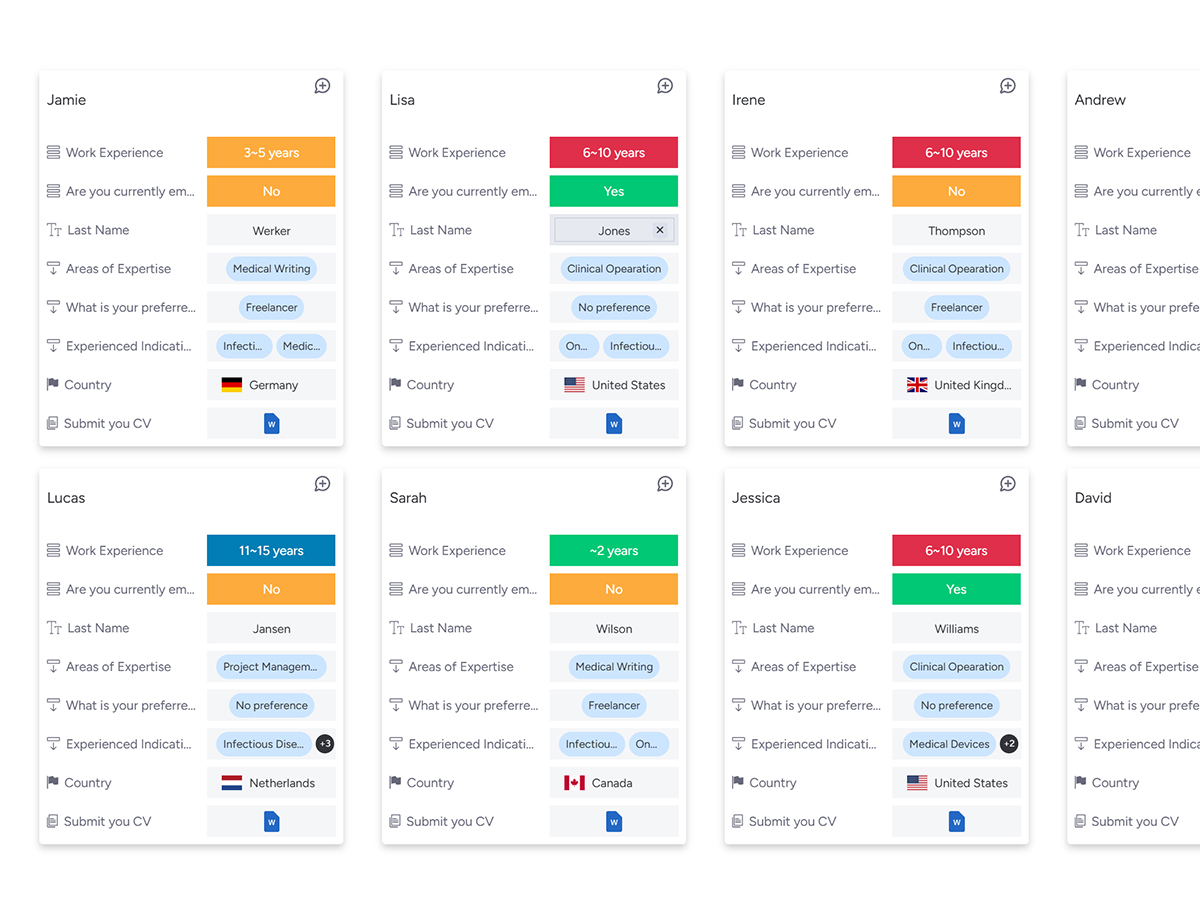
Discover experts on our platform, tailored for companies seeking specialized talent in the pharmaceutical industry.
What are the challenges in clinical trials for the pharmaceutical industry?
- Struggling with a shortage of qualified experts
- High recruitment costs in clinical trials
What is the Solution to overcome the dual challenges?
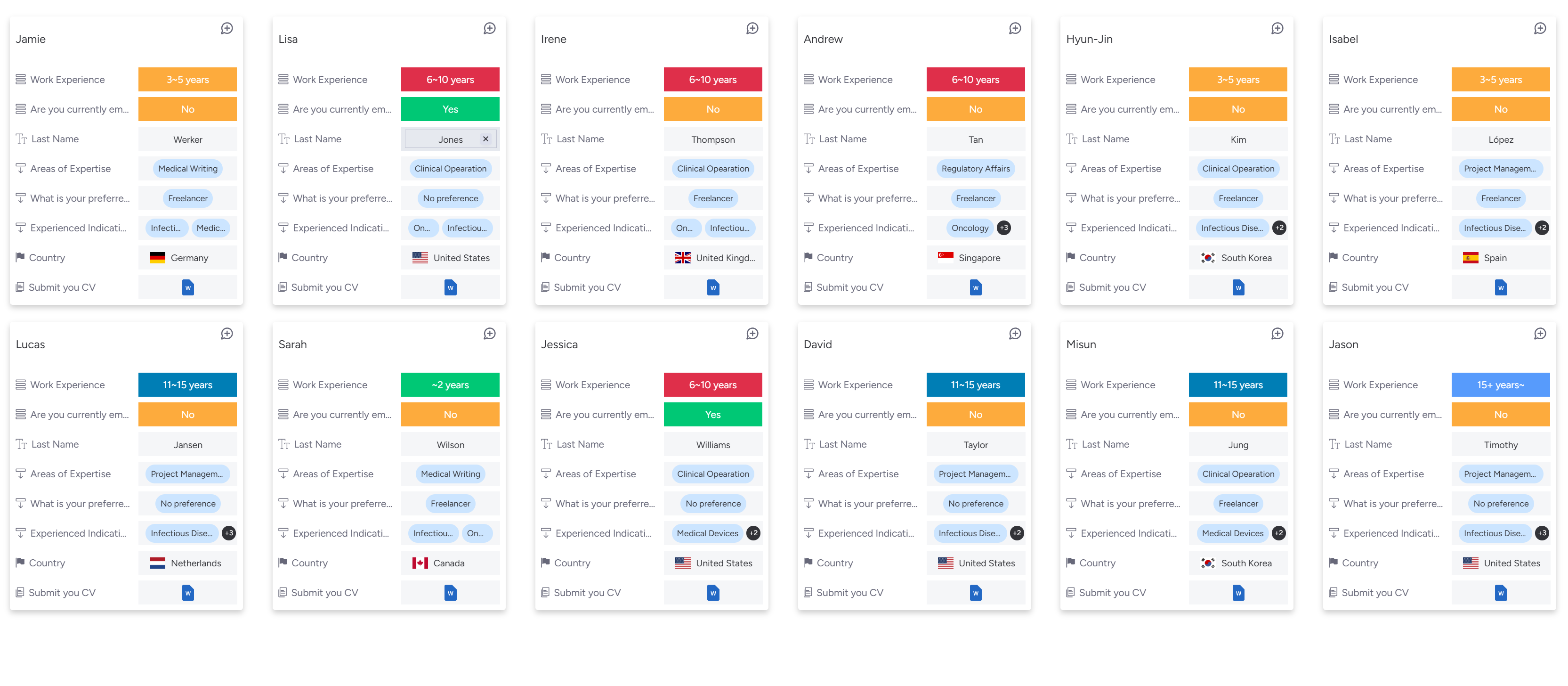
- Tailored solutions for cost savings and enhanced efficiency
- Enabling flexible cost negotiations and quick access to top professionals
Benefit - Discover the Advantage of Partnering with InnoSteP
- Access cost-effective, high-quality professional talent
- Optimize clinical trial resources, overcome pharmaceutical challenges, and accelerate business growth seamlessly.
InnoStep

- Shortage of Clinical Expertise
- High Fixed Cost Associated with Talent Recruitment
- Cost-efficient Provision of Superior Talent Through a Verified Pool of Experts
- Flexible Pricing with Custom Project Contrats

- Achieve Project Goals Through Cost-Efficient and Superior Expertise
Service Scope
Connecting top experts from various clinical specialties to meet precise trial needs with our extensive database.
Success Stroy
Successful Collaboration with Biotech Company ‘I’ in European Clinical Trials
In a strategic move, Biotech Company ‘I’ partnered with InnoSteP, which provided a Dedicated Project Manager from their pool of seasoned professionals. This collaboration marked a turning point in the company’s approach to global clinical research.

Finding a Project Manager with global research experience and expertise in European clinical trials was challenging. InnoSteP provided us with a dedicated PM who streamlined and sped up our processes, ensuring full regulatory compliance. Their proactive communication enhanced team coordination, keeping our global trials on track. I highly recommend InnoSteP for specialized staffing in global clinical trials.
Partnering with InnoSteP significantly accelerated our clinical trial site initiation, exceeding expectations. Their SSU team’s dedication, APAC regulations expertise, and excellent site management ensured all sites operated ahead of schedule.

Accelerating Clinical Trials: Overcoming Site Initiation Challenges in APAC
Pharmaceutical company 'P' struggled to speed up site initiation in the APAC region. After receiving negative responses from several CROs, they turned to InnoSteP. The dedication of InnoSteP's SSU expert team successfully accelerated their site openings.
Unlock tailored solutions with ease through our specialized registration paths



